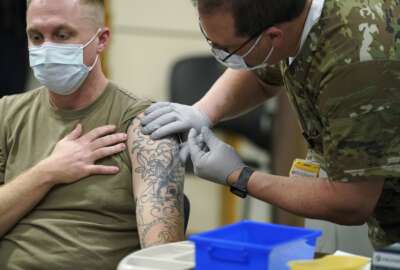
Service members and their families have a new vaccine option
In today's Federal Newscast: Service members and their families have a new option when it comes to COVID-19 vaccinations. The clock is ticking on the Small Busi...
Best listening experience is on Chrome, Firefox or Safari. Subscribe to Federal Drive’s daily audio interviews on Apple Podcasts or PodcastOne.
- Service members and their families now have a new option when it comes to COVID-19 vaccinations. The Defense Department is now offering Novavax as a preventative measure against coronavirus. The vaccine will join Pfizer, Johnson & Johnson and Moderna as options for vaccinations at military treatment facilities. Those who are unvaccinated can indicate which of the four brands they prefer when getting their shot. Novavax is not authorized for use as a booster vaccine at this time. DoD said the new vaccine was well tolerated in clinical trials. Side effects include tenderness at the injection site, headache, muscle pain and fatigue.
- The Defense Department’s Industrial Base Policy Office is starting a new pilot program aimed at leveraging private capital for manufacturing. The program will focus on advanced manufacturing for commercial and military applications that can be quickly brought to production. DoD hopes that those technologies can then be replicated in other critical technology areas. The program will run for two years manufacturing inert chemicals for munitions and agricultural purposes.
- The Energy Department is looking for help with lithium-ion battery recycling. DOE wants feedback on how federal investments can speed up the processes that go into recycling batteries, particularly in ways that align with the Biden administration’s Justice40 initiative, which directs 40% of certain federal investments to disadvantaged communities. The request for information adds $335 million to battery recycling programs, in addition to $60 million already allocated earlier this year. RFI responses are due October 14.
- Congress has to act in the next 30 days to stop an important small business program from sunsetting. The Small Business Innovation Research program will expire on September 30 unless Congress reauthorizes it. The countdown clock has been ticking all year, but federal and private sector experts said with less than 24 legislative days left , it’s critical for lawmakers to act. The hold up on this 40-year-old program is coming from Sen. Rand Paul (R-Ky.), who is concerned that companies are abusing the program. He said too many companies win awards and don’t commercialize the technology the government pays for. Sen. Ben Cardin (D-Md.) said he’s optimistic Congress will reauthorize the SBIR program by September 30.
- The General Services Administration is cracking down on third-parties acting as administrators on the SAM.gov platform. GSA said starting September 16, the role of entity administrator must be held by someone within the company like an employee, an officer or a board member. An entity administrator can no longer be a third party acting on behalf of the vendor. Eventually, GSA will convert third-parties to a data-entry role where they can create and manage entity registrations, but cannot manage and assign roles to others.
- There’s new criticism around the Pentagon’s Cybersecurity Maturity Model Certification program. The Coalition for Government Procurement said the recently released “CMMC Assessment Process,” also called the “CAP,” is too complex and confusing. The coalition is urging the Cyber Accreditation Body to withdraw, reconsider and re-write the CAP in its entirety. The document lays out how the cyber assessment process will work for as many as 70,000 defense contractors once the CMMC requirements are finalized.
- The next-generation background investigation system has hit an important milestone. The new “e-App” system for submitting security clearance forms is up and running. About 65 agencies are using the new system, intended to replace the legacy “e-QIP” system. Jeff Smith, the executive program manager for the National Background Investigation Services program, called e-App the front door to the new NBIS system. “Every case that’s submitted through the front door of NBIS is one less case that will be submitted in e-QIP. So by natural process of growth and maturity, the reliance on those legacy systems starts to go down,” Smith said. NBIS is aiming to fully replace legacy systems by next year. (Federal News Network)
- Lawmakers are asking the Office of Personnel Management for an update on plans to expand infertility coverage for federal employees. Sen. Tammy Duckworth (D-Ill.) and Rep. Gerry Connolly (D-Va.) are asking OPM to ensure all Federal Employee Health Benefits program carriers provide coverage for assistive reproductive technology (ART) starting in 2023. Those technologies include in vitro fertilization. The lawmakers said the FEHB program currently only offers limited infertility treatment, and it is often “prohibitively expensive.” OPM said earlier this year it was interested in expanding fertility coverage as part of a health equity pledge. (Federal News Network)
- After the Postal Service delivered more than 600 million of them, the Biden administration is putting an end to the delivery of free COVID tests. A message on COVIDtests.gov said the program will be suspended on September 2 because Congress has not provided additional funds to replenish the nation’s stockpile of tests.
Copyright © 2024 Federal News Network. All rights reserved. This website is not intended for users located within the European Economic Area.
Peter Musurlian
Peter Musurlian is a producer at Federal News Network.
Follow @PMusurlianWFED
Related Stories
Related Topics