

The Food and Drug Administration will begin a new hiring pilot this year in attempt to drastically cut down the time it takes to hire for mission critical posit...
Subscribe to Federal Drive’s daily audio interviews on iTunes or PodcastOne.
After fielding complaints about its lengthy and confusing hiring process — which can take anywhere from 150-to-550 days for some positions — the Food and Drug Administration is throwing the incremental approach to hiring improvement out the window.
Instead, the FDA is redesigning its hiring process, and this spring it will begin phase 1 of a brand new pilot that will attempt to resolve frustrations from agency hiring managers and its own applicants.
After all, the agency’s been fielding complaints about its lengthy and confusing hiring process from multiple parties. About 12 percent of FDA hiring managers told agency leaders that they were satisfied or very satisfied with their new hires. The rest told FDA leaders they were tired of the complexity and confused by their own roles and responsibilities in the process.
Applicants haven’t been thrilled, either. In some cases, FDA misspelled the names of their new recruits in their hiring packages.
“Getting people to come here and work for FDA isn’t the problem,” Melanie Keller, the agency’s acting associate commissioner for scientific and clinical recruitment, said in an interview with Federal News Radio. “Once they are interested, it’s the hiring process that’s so difficult to get them here. Many candidates give up halfway through.”
It’s part of a multi-pronged approach to address multiple pain points and modernize the hiring system overall at the agency.
Like many applicants to federal positions, FDA applicants themselves were also frustrated by the lack of communication from the agency throughout the hiring process. Of 657 new FDA hires in fiscal 2016, 34 percent said they were satisfied with the overall hiring experience, according to an agency report on the topic.
The report, which FDA publicly released in November, came after the agency conducted surveys, reviewed current hiring procedures and held focus groups with managers and new recruits. FDA is also collecting suggestions from the public on its hiring practices.
“[With] the idea of transparency, discussion and feedback — something that has worked for us extremely well in the scientific area —now we’re bringing those principles into an operations area,” Rachel Sherman, FDA’s principal deputy commissioner of food and drugs, said during a November public meeting on the agency’s hiring reforms. “There’s nothing more important to FDA than our workforce and being able to serve the public. We’ve grown very rapidly and like many bureaucracies, our processes have not kept up with our growth.”
Other departments and agencies certainly aren’t immune to challenges with the federal hiring process. The Office of Personnel Management spent much of 2016 educating agency hiring managers across government on existing authorities, flexibilities and tools they can use to hire better qualified talent more quickly.
But the topic got greater attention from former Health and Human Services Secretary Tom Price, who identified hiring as a challenge for multiple HHS agencies and subcomponents under the department’s “Reimagine HHS” initiatives.
The pilot is also the result of a directive from FDA Commissioner Scott Gottlieb, who asked that the agency look at five aspects of the hiring process that caused the most pain for managers and applicants: timeliness, accuracy, quality, customer satisfaction and employee satisfaction.
“We’re building the new process around those five dimensions,” Keller said. “We want to make sure that the HR staff have the tools and skills they need to do their job, and if there’s a problem, there’s real time escalation, and at the end of the day, we’re getting the right candidates in the right roles faster.”
The agency will start the pilot at the Center for Drug Evaluation and Research (CDER) and the Center for Biologics Evaluation and Research (CBER), two user-fee-funded centers within FDA. The pilot will run through 2018 or until FDA fills all of the 140 vacancies within CDER and CBER, Keller said.
During that time, FDA will test its new ideas, fine tune them and map a path forward to implement them throughout the rest of the agency.
For FDA, improving its time-to-hire is the top priority. Not all vacancies take 550 days to fill, but biomass statisticians, pharmacologists and toxicologists are among the positions that are most difficult to address, Keller said.
To address the timeliness challenge, FDA mapped its entire hiring process from beginning to end.
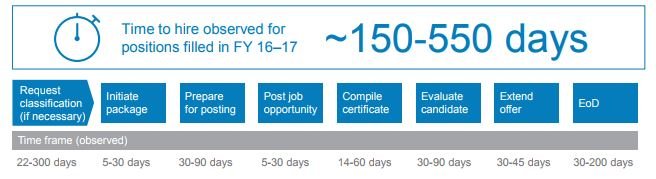
Under the pilot, FDA human resources leaders will set concrete deadlines for each step of the hiring process. If one office becomes too backlogged with work, a new IT dashboard will help HR supervisors and hiring managers identify the hold-up.
The current process does not have a transparent and agile user-friendly HR system, so managers have no way to track their actions, Keller said.
FDA will soon issue a contract for a new IT dashboard, which hiring managers can use to view where their recruits are in the process.
“It’s sort of like when you go to order a Domino’s pizza; you know exactly where it is in the process,” she said. “This system will enable us to see where the recruit is. Is it in classification? Is the vacancy announced? Who has the current action? One of the pieces that we’ll also be able to track is of all of the talent analysts working on a given HR action, what’s their workload like? One of the issues is we have tapped-out HR staff currently, so through this IT tool, we’ll be able to monitor and balance the workload as well.”
In addition, the agency concluded that too many people are involved in each stage of the hiring process.
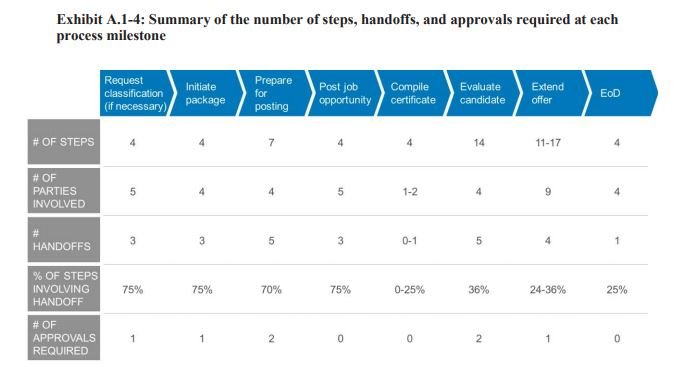
“There are a lot of different people that are in the hiring process, and there’s too many ‘cooks in the kitchen,’ Keller said. “We’re trying to have a new process where the HR specialist or the talent strategy officer is working directly with the FDA hiring manager, and there are not multiple people between them. When there’s too many people in a process, there’s too much back and forth [and] there’s too much mis-advice or opportunities for mis-counsel, and so we’re connecting the hiring manager directly with a person that’s providing that hiring support.”
Despite continuing resolutions and hiring freezes, hiring at FDA has been relatively steady, Sherman said.
“It’s not enough,” she said. “We want to do more. We want to do better. We don’t just want to hire by brute force. We want a modern system that will meet our needs not only now but into the future.”
Copyright © 2024 Federal News Network. All rights reserved. This website is not intended for users located within the European Economic Area.
Nicole Ogrysko is a reporter for Federal News Network focusing on the federal workforce and federal pay and benefits.
Follow @nogryskoWFED


