

The Food and Drug Administration has launched a new brand and advertising campaign to attract and recruit new employees.
For Washington-area federal employees who braved Metro this month, they may have seen tangible evidence of the Food and Drug Administration’s latest efforts to improve its recruitment and hiring efforts.
FDA launched a new recruitment “brand” and advertising campaign this summer, and it’s part of multi-pronged approach to fill some 1,400 vacancies at the agency with more speed and less stress.
The agency’s message is simple, and it hearkens back to common adage federal managers mention when they discuss their mission and recruitment challenges: You want to make a difference. We can help you make that difference.

“We conducted some internal surveys to try to figure out what marketing campaign would work best,” Melanie Keller, FDA’s acting associate commissioner for clinical and scientific recruitment, said in an interview. “We learned that most new employees came to the FDA because of our mission.”
The new ad campaign is the most noticeable piece to FDA’s new recruitment strategy. But behind the scenes, the agency also updated information about its jobs on its own website and started an @FDAJobs Twitter account and LinkedIn page. It also created new videos to explain the application process and have more planned.
FDA has struggled to attract candidates to their positions in the past, Keller said. Usually, most applicants interested in FDA’s jobs are actively searching and applying for multiple positions on USAJOBS.gov. They’re not “passive job seekers.”

“What we find is some of our best candidates who come in the door, especially in the executive ranks, are happy in their jobs or maybe close to retiring in the private sector and might have an interest at FDA,” she said. “We’re trying to seek out those candidates through executive search or our search committees or getting opportunities in front of them, because they’re not going to go to USAJOBS.”
FDA’s hiring system has long been a point of frustration for the agency’s hiring managers and supervisors. It some cases, it took the agency as many 550 days to fill certain positions.
FDA last year collected feedback from hiring managers, leaders and applicants themselves about the process. In many cases, the feedback wasn’t particularly glowing. Keller and her team discovered that not only did it take too long, but the hiring process had too many steps and people involved. One person didn’t have complete responsibility for a new hire, and supervisors were left in the dark about the status of potential applicants.
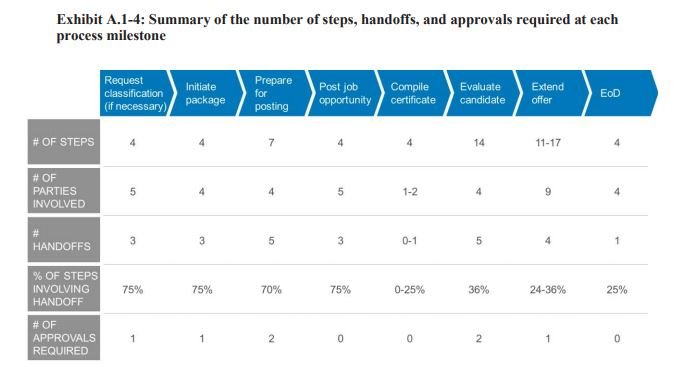
The agency has since revamped and eliminated unnecessary steps and hands from the hiring process. The goal, Keller said, is to recruit, hire and onboard a new employee to FDA between 80-to-140 days.
To prepare for the hiring pilot’s launch, FDA brought on new staff and began to train those new employees.
It also created a “talent academy” to train those employees on federal and FDA-specific hiring rules, customer service and IT and technical skills.
“When you hire staff into a particular unit in the government, they come to you from different places and they come to you with different levels of expertise,” Keller said. “We wanted to make sure that every single person working in the hiring pilot had robust training and technical expertise.”
The agency started advertising jobs under this new pilot back in June, starting with regulatory health project managers, regulatory counsels, computer scientists and operation research analysts, Keller said. FDA fully launched the pilot on July 30 with 24 positions.
In addition, FDA is close to deploying a new hiring and talent tracker. Keller likened the new HR system to a “pizza tracker,” where supervisors can see exactly where each recruit is at each point in the hiring system.
Keller’s team has also spent the past year developing a new, alternative personnel system to help FDA more easily attract top talent for 38 high-demand occupations.
To develop the new pay system, they spoke with other agencies, such as the Securities and Exchange Commission and Federal Aviation Administration, that had developed their own alternatives in the past.
The mandate is part of the 21st Century Cures Act, which Congress passed back in December 2016. The law gives the agency authority to develop its own alternative pay system for certain positions and enables FDA leadership to set annual salaries up to a maximum of $400,000.
“Of course, we’ll never be able to pay many of our staff what private industry does,” Keller said. “We don’t have the budget, nor would it make sense to do that. The Cures pay system really tries to get us closer.”
FDA so far has used the new personnel system to hire two new employees. Keller said she’s focusing on using the new system to hire more supervisors and leaders in the coming months. Recruiting new talent to leadership roles is especially important for FDA, as nearly half of FDA senior leaders are eligible retire by fiscal 2020.
“The stats are a bit scary,” Keller said. “We knew that the baby boomers would eventually retire; they didn’t quite retire when we thought they would. What we find though is that even though senior leaders are eligible to retire, they’re, on average, staying between six-to-seven years longer than their eligibility, so that’s good news for us.”
Copyright © 2025 Federal News Network. All rights reserved. This website is not intended for users located within the European Economic Area.
Nicole Ogrysko is a reporter for Federal News Network focusing on the federal workforce and federal pay and benefits.
Follow @nogryskoWFED


