
GAO offers advice for growing pains at FDA headquarters
The Food and Drug Administration's headquarters in White Oak, Maryland needs some work to address cramped office space and a lack of parking spots. But government...
The Food and Drug Administration oversees consumer safety of everything from animal food to Zika vaccines, but when it comes to managing its own facilities and workforce, the agency raises some red flags.
A new report from the Government Accountability Office finds that FDA’s headquarters at the White Oak campus in Maryland, about 40 minutes north of Washington, D.C., is not up to standard for its high-risk status, and the agency’s plans to address cramped offices and parking spaces need more detail before they’re put into action.
More than 10,000 people work in 3.8 million square feet of space at the White Oak campus, and FDA is proposing to add another 5,900 people to the site by 2020.
The 2009 White Oak master plan called for 10 office buildings, 3 labs, 5 support facilities, and 5 parking garages.
“As of 2016, the campus was partially complete, with 9 of the 10 planned office buildings constructed and 27 percent more staff than the 8,297 staff planned for in the completed buildings. Benefits of the consolidation cited by FDA officials included increased collaboration and improvements in efficiency from factors such as co-located staff and shared labs,” GAO said. “FDA has taken steps to plan for the future of the White Oak campus, but its planning efforts lack some elements of leading practices for facilities planning.”
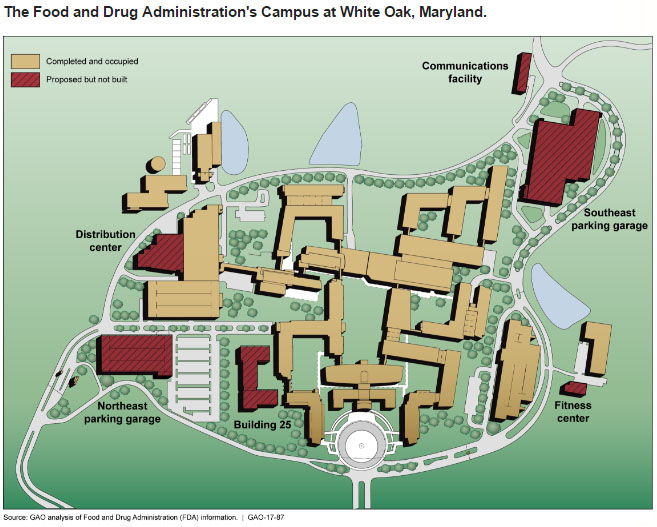
Federal auditors recommended three actions for FDA to take: implement vehicle access control measures to meet high-risk facility standards, explain more clearly the connection between FDA’s strategic and facilities plans, and collect and document more information about the daily operations of the campus.
Jim Esquea, assistant secretary for legislation at the Department of Health and Human Services — which oversees FDA — said in a November 2016 letter that the department concurred with GAO’s recommendations and was in the process of implementing them.
Vehicle separation system
The Homeland Security Department in 2014 deemed the White Oak campus a “high-risk facility” because of its size, the number of staff on campus and the nature of the laboratory work at the site.
According to DHS’ Interagency Security Committee, a high-risk facility needs to meet certain security standards, including “controlled vehicle access and parking.” the report stated.
“Due to traffic and parking concerns and a desire not to slow the flow of vehicles into parking garages, FDA has not implemented vehicular access controls, such as perimeter vehicular barriers, card access drive-on gates, and forced separation of visitor and employee parking,” GAO stated. “Two of the five parking garages planned in the 2009 master plan were not built, and would have provided 3,297 permanent parking spaces. Due to the construction of temporary surface parking lots, officials told us that the campus has reduced the deficit of parking spaces to 940 spaces, compared to the planned inventory of parking spaces of 6,926.”
While conducting the report, GAO learned that some employees were moving their schedules so they could get to the campus early and find a parking spot. Others told the auditors they were reluctant to leave the campus to attend mid-day meetings or visit other FDA offices because they were concerned about finding a parking spot when they returned to White Oak.
Esquea said FDA was implementing a three-part plan to address campus access controls. The first phase began in November 2016 with the redirection of all campus visitors out of employee parking areas located within security boundaries.
Phase 2 also started in November and tested Fast Pass lanes ant security gate traffic lights.
“Phase 3 is planned to begin in mid-December, at which time security gate arms will be activated,” Esquea said. “Only vehicles with active Fast Pass or employees with contractors/visitors with a valid PIV badge will be allowed to cross the campus security perimeter and park in the designated employee parking areas. We anticipate full implementation by the end of January 2017.”
Linking mission and facilities
Finding an open parking spot is just one of the issues facing staff on the crowded campus.
FDA permitted telework for some employees, while others share office space to save on work room. While the former has improved work-life balance, GAO said in its report, FDA staff said it can be hard to collaborate with a team that’s not all under the same roof.
Staff also told auditors that shared office space and use of cubicles “has been challenging for those staff who routinely handle proprietary information related to drug applications, or those who handle sensitive personnel related tasks.”
The lack of an official distribution center — the result of a high price tag — has pushed deliveries and supplies into an underground tunnel.
“According to officials, the service tunnel system was not designed for this purpose and is overcrowded, thereby potentially creating a safety and security hazard,” GAO said. “Officials reported that the construction of the distribution center is a high priority for FDA as it plans for the future of the White Oak campus.”
The problem with those plans for the future, GAO said, is that they don’t yet include “key elements of leading capital and strategic facilities planning practices,” which means the plans might not actually meet the needs of FDA and its mission.
While the agency outlined that it wants to carry out its mission to protect and advance public health and safety, FDA doesn’t explicitly lay out how its future facility plans will do that, GAO said.
Esquea said FDA will incorporate those links in its fiscal 2018 Strategic Facilities Plan.
Daily operations
GAO also recommended that FDA collect more information about its daily campus population, office usage, parking availability and infrastructure capacity.
“The scopes of work for FDA’s proposed planning efforts state generally that data should be collected in areas where adequate data do not exist, are unverifiable, or insufficient,” GAO said. “As a result of these current data limitations, FDA’s facilities planning efforts offer limited assurance that recommendations developed for the future of the White Oak campus will accurately represent the full scope of facility needs and account for all current and future performance gaps.”
Esquea said FDA will incorporate information on employees and visitors at the White Oak site, along with the number of cars.
Copyright © 2025 Federal News Network. All rights reserved. This website is not intended for users located within the European Economic Area.





