

After earning its own authority from Congress, the Food and Drug Administration is developing an alternative pay and personnel system for 38 occupations.
The Food and Drug Administration is developing a new alternative pay system in an effort to better meet growing demands on its people and mission — and to meet a mandate from Congress.
The mandate is part of the 21st Century Cures Act, which Congress passed back in December 2016. The law gives the agency authority to develop its own alternative pay system for certain positions and enables FDA leadership to set annual salaries up to a maximum of $400,000.
FDA identified 38 occupations to include in the new pay system. The agency will base a new pay structure on the General Schedule scale and will include nine bands, FDA Commissioner Scott Gottlieb said in the agency’s latest workforce assessment, which FDA presented to Congress last month. That assessment was also required under the Cures Act.
“It specifically addresses those staff in scientific, technical and professional positions whose work supports the development, review and regulation of medical products,” Gottlieb said of the new pay authority. “However, this focus may not fully address other hiring needs across FDA. The agency is committed to working further on these challenges.”
FDA said it used the Cures Act authority to make two new hires so far in early 2018 and expects more in the coming months.
The agency created a working group and steering committee to discuss how it could implement an alternative pay system authorized in the Cures Act. The groups also reviewed other alternative pay systems throughout government to guide their work.
The new personnel system isn’t ready yet, as FDA is currently completing policies and procedures to determine where employees within the 38 occupations would land within the pay structure.
“The next phase of implementation will focus on mission-critical positions that have historically been difficult to fill, and where FDA research has identified disparities between FDA salaries and market rates,” Gottlieb wrote.
With the Cures Act, FDA estimates it will need roughly 200 more employees to implement congressional requirements in regenerative medicine, breakthrough devices, qualification of biomarkers and other responsibilities.
But the agency has long struggled to quickly recruit and hire top talent, a challenge that may become prevalent as FDA faces an influx of retiring leaders.
“It is well known that FDA has had challenges recruiting and hiring,” Gottlieb said. “This is, in large measure, due to the cumbersome patchwork of hiring authorities that were not designed specifically for FDA. The existing patchwork of hiring authorities created inconsistencies across positions and centers and hiring inefficiencies across the entire FDA.”
FDA hiring managers were burdened by too many hiring authorities, confusion that contributed to the agency’s average time-to-hire, which ran anywhere from 150-to-550 days for some positions.
But with a specific hiring and personnel authority targeted only for FDA, the agency believes it will, eventually, turn things around.
FDA’s approach may be one worth watching in the future, as the Trump administration has expressed an interest in creating specific personnel systems for different kinds of occupations.
The agency also faces a potential leadership vacuum, as nearly half of FDA senior leaders are eligible retire by fiscal 2020, Gottlieb said.
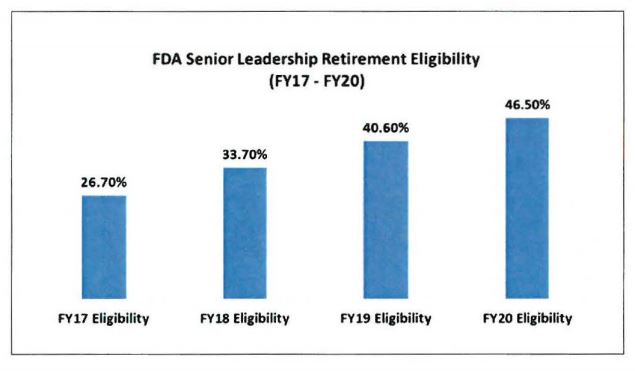
“Several organizational components are in a moderate or high risk status because 30 percent or more of the senior leaders (executive level and General Schedule 15) became eligible to retire as of Sept. 30, 2017,” FDA wrote.
FDA has also struggled to recruit and retain supervisors, who generally told the agency that the small bumps in salary aren’t enough to take on the additional responsibilities of being a manager. And because FDA has used a patchwork of hiring and pay authorities to recruit new employees in the past, some managers end up supervising employees with higher salaries, the report said.
“Agency supervisors report that as employees are trained at FDA and gain regulatory experience in their fields over two or more years, they become extremely attractive to industry and other private entities, which are sometimes able to attract such employees away from the agency with higher salary and benefits packages,” the agency wrote. “Losing employees in this manner represents the loss of a significant investment in time and other resources and creates a lag in full productivity when a new person is hired for a vacated position.”
Pay in general has been another challenge, as the private sector can offer significantly higher salaries.
The Cures Act also gave FDA the authority to simplify and expedite hiring process for certain positions.
The agency kicked off a hiring pilot in the spring, with the intention of filling about 140 vacancies within the Center for Drug Evaluation and Research (CDER) and the Center for Biologics Evaluation and Research (CBER), two user-fee-funded centers within FDA.
Ultimately, the agency hopes to average roughly 80 days to hire a new employee to one of these centers, Gottlieb said.
Human resources staff are getting new training requirements and opportunities to improve their own performance.
FDA is also implementing a new electronic classification system to streamline the administrative process and improve communications between HR staff and hiring managers.
Copyright © 2025 Federal News Network. All rights reserved. This website is not intended for users located within the European Economic Area.
Nicole Ogrysko is a reporter for Federal News Network focusing on the federal workforce and federal pay and benefits.
Follow @nogryskoWFED


